
Lithium-ion batteries that are resistant to exploding or catching fire have been developed by scientists.
The devices produced sufficient energy for use in household electronics, but did not ignite - even when punctured repeatedly with a nail.
The batteries use a water-salt solution as their electrolyte, removing the risks carried by some non-aqueous commercial models.
The research is published in the journal Joule.
"In the past, if you wanted high energy, you would choose a non-aqueous lithium-ion battery, but you would have to compromise on safety. If you preferred safety, you could use an aqueous battery such as nickel/metal hydride, but you would have to settle for lower energy," said co-author Kang Xu, from the US Army Research Laboratory (ARL).
"Now, we are showing that you can simultaneously have access to both high energy and high safety."
The latest paper follows on from a 2015 publication in Science journal, in which the same team unveiled a similar 3.0 volt battery with an aqueous electrolyte.
However, at the time, the researchers were prevented from reaching higher voltages by something called "cathodic challenge". This occurs when one end of the battery (the anode) - made from graphite, or lithium metal - is degraded by the water-based electrolyte.
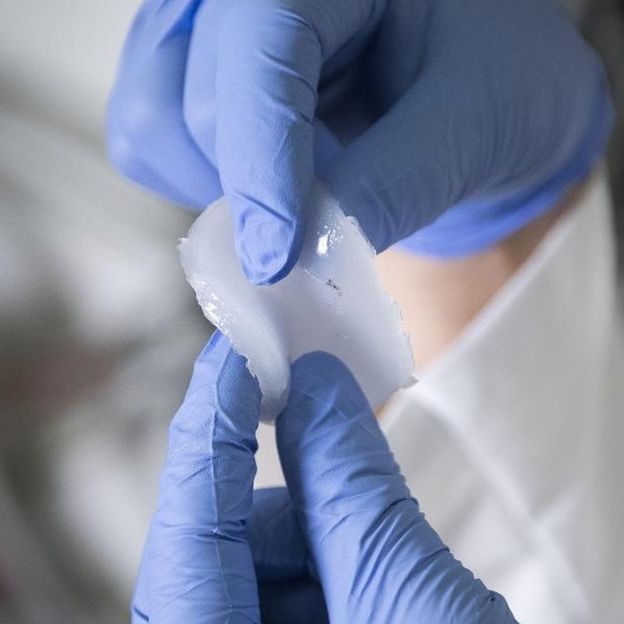
The gel polymer decomposes on the battery's first charge to form a stable layer called an "interphase". This interphase protects the anode from chemical reactions that stop it from working properly and allows the most desirable anode materials to be used in the battery.
By coating the anode with the protective gel polymer, the scientists were able to push the battery voltage up to 4.0, making it useful for household electronic devices such as laptop computers.
The addition of the gel coating also boosts the safety advantages of the new battery when compared to standard non-aqueous lithium-ion batteries. It also boosts the energy density when compared to other proposed aqueous lithium-ion batteries.



