

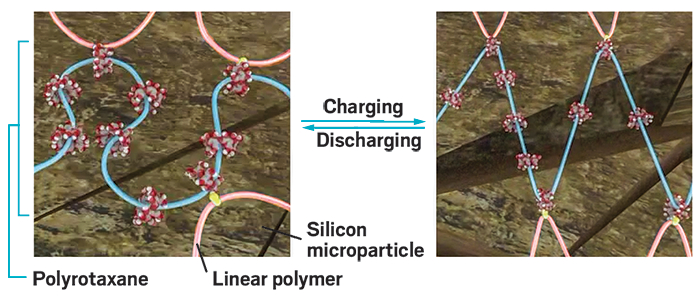
Replacing the anode in commercial lithium-ion batteries with a silicon-based version could improve how much charge a battery can hold. But silicon anodes have been difficult to develop because they massively expand and contract as a battery charges and discharges, causing particles in the anode to break apart and the battery to fail.
The researchers devised a unique polymeric network made of the conventional linear polymer polyacrylic acid covalently linked to polyrotaxanes containing mechanical bonds. In the polyrotaxanes, an amine functionalized polyethylene glycol chain is threaded through a number of cyclodextrin rings. During battery charging, as the silicon anode expands, the rings freely slide along the chain to dissipate stress, operating like a pulley system. The researchers showed that the molecular netting demonstrated excellent elasticity, withstanding up to about 400% strain.
The two main advantages of using this polymeric network are cost and processability, Coskun says. The team was able to make anodes with silicon microparticles, which are easier and cheaper to make in large quantities than the smaller and more commonly studied silicon nanoparticles, he says. Also, the new polymer accounts for only 10% by weight of the anode, compared with the 20% polymer binder typically used in silicon anodes.
The researchers are currently collaborating with a major battery maker to test their polymeric pulley system on real battery products.